‘Especially concerning’: FDA issues warning over CBD product
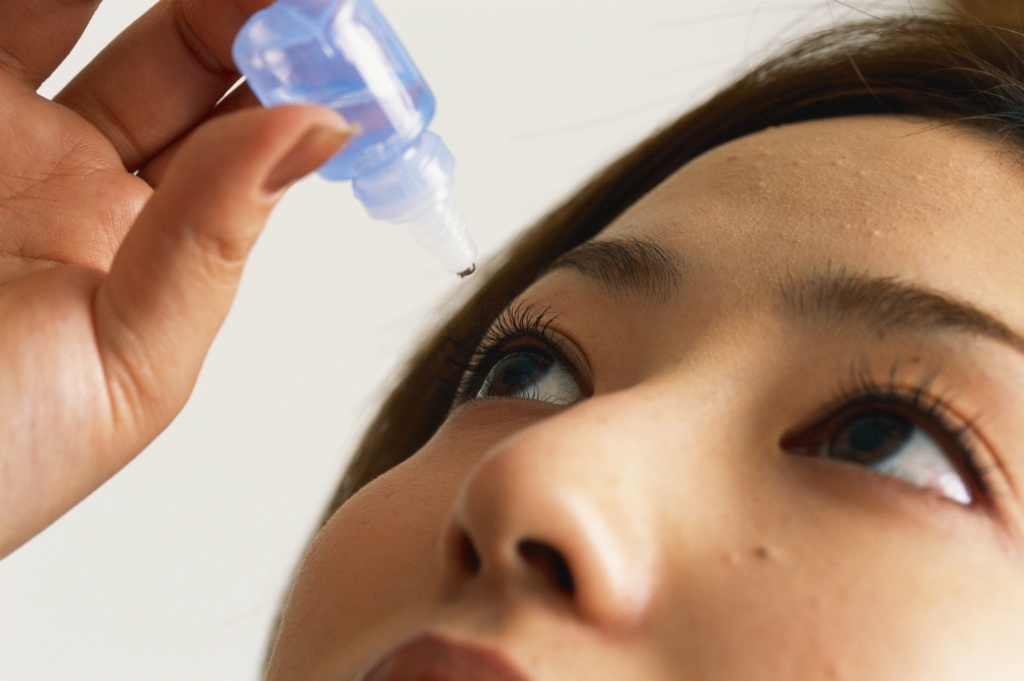
CBD has experienced a surge in popularity in recent years. While gummies, tinctures, and drinks may dominate sales, other niche products have also debuted (remember CBD pillowcases?). However, the Food and Drug Administration (FDA) has approved only one CBD-derived drug, meant to treat a severe seizure disorder. Recently, the FDA sent a warning letter to one company over its “especially concerning” product: CBD eye drops.
On August 25th, the FDA sent the grievance to Arizona-based Trew Balance. The product in question is called Trew Vision CBD Eye Drops. The government believes the company is violating the law, arguing it’s an “unapproved new drug.”
RELATED: Questionable additive leads to weed vape recall
The FDA cited the brand’s website as evidence, saying the product is intended to treat certain conditions. The letter points to a product page, which has since been deleted, that claimed the drops fought pink eye and sore eyes while protecting against things like macular degeneration and computer radiation exposure.
Government officials were alarmed at the delivery mechanism of the drops, which may be more hazardous than other unregulated CBD products on the market.
“Your…product is especially concerning from a public health perspective,” the letter said. “Ophthalmic drug products, which are intended for administration into the eyes, in general pose a greater risk of harm to users because the route of administration for this product bypasses some of the body’s natural defenses.”
RELATED: New York cannabis ruling could have far-reaching implications
In addition to marketing the product, the FDA also said engaging in interstate commerce with an unapproved drug is a violation of the law. The feds also took issue with the brand labeling the drops as a “homeopathic drug.” The product is not recognized in the Homeopathic Pharmacopoeia of the United States (HPUS) and therefore, should not be labeled as such.
Representatives for Trew Balance told GreenState via email that the CBD eye drops came from a 3rd party manufacturer that provided the brand with what they believed to be “proper documentation” for the product. The representative also said they reached out to the manufacturer in question before the FDA letter was sent and found they were no longer in business, ceasing the sale of the product several months ago.
“All of the concerns of the FDA were addressed, and Trew Balance is in compliance and has been in compliance for decades,” the representative said.
The letter serves as a warning to brands to exercise extreme caution in the types of products they make—and how they market them. While CBD has shown great promise as a potential treatment for a range of health conditions, from inflammation to infection, it may not be a great idea to put it in your eyes.
*This article was updated on September 8th, 2025, to include a comment from Trew Balance.
